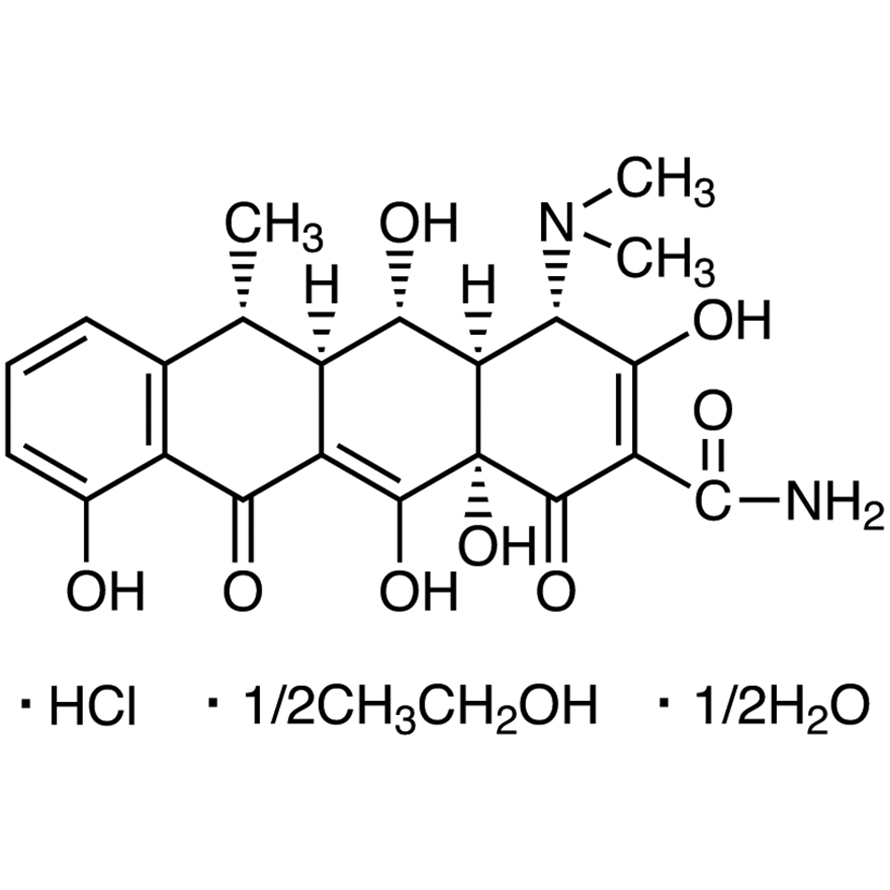
To understand doxycycline hyclate’s mechanism, begin by visualizing its core structure: a tetracycline antibiotic featuring a four-ring naphthacene system. This system, with its specific hydroxyl and amino groups, directly interacts with bacterial ribosomes.
The hyclate salt form, specifically, adds a counterion–hyclate–improving the drug’s solubility and bioavailability. This means your body absorbs and utilizes doxycycline hyclate more effectively than the free base. Observe the resulting structural modifications affecting its absorption and distribution within the body. Note the impact of these changes on its pharmacokinetic profile.
The presence of specific functional groups within the tetracycline core dictates its broad-spectrum activity against a range of bacteria. Examine the spatial arrangement of these groups; their precise positioning is directly responsible for the molecule’s binding affinity to the bacterial 30S ribosomal subunit, inhibiting protein synthesis and ultimately, bacterial growth. Specific modifications on the molecule impact this binding.
Remember: Detailed structural analysis, using techniques like X-ray crystallography or NMR spectroscopy, reveals the precise three-dimensional arrangement of atoms. This detailed understanding is crucial for drug development and understanding potential interactions with other medications or biological molecules.
- Doxycycline Hyclate Structure: A Detailed Look
- Doxycycline Core Structure
- Hyclate Counterion’s Role
- Structural Implications for Pharmacokinetics
- Conformation and Isomerism
- Chemical Formula and Molecular Weight
- Calculating Molecular Weight
- Impact of Hydration
- Structural Components: Tetracycline Core and Hyclate Moiety
- Conformation and Isomerism
- Isomerism: A Closer Look
- Impact on Drug Action
- Crystalline Structure and Polymorphism
- Impact of Structure on Pharmacological Properties
- Spectroscopic Characterization (e.g., NMR, IR)
Doxycycline Hyclate Structure: A Detailed Look
Doxycycline hyclate consists of a doxycycline molecule bound to a hyclate counterion. This counterion, typically a chloride ion, significantly impacts solubility and stability.
Doxycycline Core Structure
The doxycycline molecule itself is a tetracycline antibiotic. Its core structure includes four fused rings: A, B, C, and D. Key features include:
- A dimethylamino group at position 4.
- A hydroxyl group at position 6.
- A keto group at position 11.
- A dimethylated amino group at position 12.
These functional groups directly affect its antimicrobial activity by interacting with the bacterial ribosome. Slight variations in these groups lead to different tetracycline derivatives with varied properties.
Hyclate Counterion’s Role
The hyclate counterion modifies doxycycline’s physicochemical properties. Chloride (HCl) enhances its solubility in water, improving its bioavailability. Other counterions are possible, affecting dissolution and absorption rates. The counterion does not interfere directly with the antibiotic’s mechanism of action.
Structural Implications for Pharmacokinetics
- Solubility: Hyclate salts increase aqueous solubility compared to the free base, leading to better absorption.
- Stability: The counterion helps to protect doxycycline from degradation in various environments.
- Formulation: The choice of counterion significantly impacts the type of pharmaceutical formulation, influencing dosage form (e.g., capsule, tablet).
Understanding the detailed structure of doxycycline hyclate–both the doxycycline molecule and its associated counterion–is critical for comprehending its pharmacokinetics and ultimately, its therapeutic efficacy.
Conformation and Isomerism
Doxycycline exists in different conformational isomers, impacting its activity. These conformations relate to rotations around specific bonds within the tetracycline core. Structural analysis, often involving X-ray crystallography, determines the precise conformation present in various forms of doxycycline hyclate. This information is useful for rational drug design and improving stability.
Chemical Formula and Molecular Weight
Doxycycline hyclate’s chemical formula is C22H24N2O8·HCl·H2O. This reflects its composition: 22 carbon atoms, 24 hydrogen atoms, 2 nitrogen atoms, 8 oxygen atoms, one molecule of hydrochloric acid (HCl), and one water molecule (H2O).
The molecular weight, calculated from this formula, is approximately 496.42 g/mol. This value is crucial for various applications, including dosage calculations and pharmaceutical formulation.
Calculating Molecular Weight
The calculation involves summing the atomic weights of each element, considering the number of atoms of each. Atomic weights can vary slightly depending on the isotopic composition, but generally accepted values are used for these calculations. You can find these values in periodic tables.
Impact of Hydration
Note the inclusion of a water molecule (H2O) in the formula–this represents the hyclate form of doxycycline. This hydration significantly influences the molecular weight and the physical properties of the compound.
| Element | Atomic Weight (g/mol) | Number of Atoms | Total Weight (g/mol) |
|---|---|---|---|
| Carbon (C) | 12.01 | 22 | 264.22 |
| Hydrogen (H) | 1.01 | 26 | 26.26 |
| Nitrogen (N) | 14.01 | 2 | 28.02 |
| Oxygen (O) | 16.00 | 8 | 128.00 |
| Chlorine (Cl) | 35.45 | 1 | 35.45 |
| Total | 481.95 |
The table above shows a simplified calculation. Remember to use the most accurate atomic weights available for the most precise molecular weight determination.
Structural Components: Tetracycline Core and Hyclate Moiety
Doxycycline hyclate’s structure features two key parts: the tetracycline core and the hyclate moiety. The tetracycline core comprises four rings (A, B, C, and D) fused together, each with specific functional groups influencing its activity. These groups include a ketone at C-11, dimethylamino group at C-4, and hydroxyl groups at C-6, C-10, and C-12.
The hyclate portion, specifically the chloride salt of the hydrate, modifies the overall molecule’s properties. It’s a hydrated hydrochloride salt, meaning a water molecule and a chloride ion are bound to the tetracycline core. This salt formation enhances the compound’s stability and solubility, improving its bioavailability and pharmaceutical applications. The chloride ion interacts with the amine group, improving the molecule’s stability.
Key differences exist between doxycycline hyclate and other tetracyclines due to this hyclate moiety. For example, enhanced solubility directly impacts drug delivery and absorption. Understanding these structural details is vital for comprehending its unique pharmacological profile. Careful consideration of this detailed structure is crucial for understanding drug interactions and metabolism.
Analyzing the precise arrangement of atoms within these components is essential for drug development and research. Structural modifications, even minor ones, can significantly affect the drug’s efficacy and safety profile.
Conformation and Isomerism
Doxycycline hyclate exists as a mixture of conformers due to the flexibility of its tetracycline core. Rotation around single bonds allows for various spatial arrangements. These conformational changes don’t alter the atom connectivity but affect the molecule’s overall shape and, consequently, its biological activity and interactions. Observe how different conformations influence binding to bacterial ribosomes. Understanding these conformational changes is vital for designing derivatives with improved pharmacological properties.
Isomerism: A Closer Look
Doxycycline hyclate also exhibits isomerism. Specifically, it possesses chiral centers, leading to stereoisomers (enantiomers and diastereomers). The specific configuration of these chiral centers significantly impacts its potency and bioavailability. Pharmaceutical preparations typically use a racemic mixture, meaning both enantiomers are present in equal amounts. However, research indicates that one enantiomer might be more effective or have a different metabolic profile than the others. Further investigation into individual isomeric activities is needed for the development of more specific and efficient drugs.
Impact on Drug Action
Note: The precise conformational preferences and the influence of isomerism on doxycycline hyclate’s interaction with its target are complex areas actively being researched. Current studies focus on this to better design and produce more effective antibiotics.
Crystalline Structure and Polymorphism
Doxycycline hyclate exists in various crystalline forms, each exhibiting unique properties. These polymorphs differ in their packing arrangements of molecules within the crystal lattice, impacting physical characteristics like solubility, dissolution rate, and stability.
The most common form is the monohydrate, characterized by a specific arrangement of water molecules within the crystal structure. This hydration affects its stability and hygroscopicity – its tendency to absorb moisture from the air. Anhydrous forms also exist, lacking this bound water. These anhydrous forms often display enhanced stability compared to the monohydrate but might exhibit different dissolution profiles.
Specific crystallographic data, including unit cell parameters and space group designations, are available in scientific literature and databases like the Cambridge Structural Database (CSD). Consult these resources for detailed structural information on specific doxycycline hyclate polymorphs. Analyzing diffraction patterns – such as powder X-ray diffraction (PXRD) – allows identification of the different polymorphs.
The understanding of these polymorphic variations is critical for pharmaceutical development and formulation. Manufacturing processes influence the resulting polymorph, directly affecting the drug product’s quality, efficacy, and shelf life. Careful control of crystallization conditions is necessary to obtain the desired polymorph with optimal properties for drug delivery.
Further investigation into the relationship between crystalline structure and bioavailability remains an area of active research. Studies using techniques like solid-state nuclear magnetic resonance (SSNMR) provide valuable insights into molecular mobility and interactions within the crystal lattice which influences drug release.
Impact of Structure on Pharmacological Properties
Doxycycline hyclate’s unique structure directly influences its pharmacological activity. The presence of a dimethylamino group enhances its lipophilicity, facilitating better absorption across cell membranes and contributing to its broad tissue distribution. This structural feature also allows for efficient penetration of the bacterial cell wall.
The tetracycline ring system itself is critical for binding to the bacterial 30S ribosomal subunit. This interaction inhibits protein synthesis, a mechanism central to doxycycline’s antibacterial effect. Slight modifications to this ring system, however, can significantly alter its potency and spectrum of activity.
The hyclate salt form increases doxycycline’s water solubility, improving its bioavailability and reducing gastrointestinal irritation compared to the free base. This salt formation is a key factor in the drug’s formulation and delivery.
Specific structural features dictate its susceptibility to degradation. For instance, the presence of certain functional groups makes it prone to photodegradation, highlighting the need for storage in dark, dry conditions. Careful consideration of these structural features guides formulation strategies to enhance stability and therapeutic efficacy.
Finally, understanding the structure-activity relationship allows for the design of potential doxycycline analogs with improved properties, such as enhanced activity against specific bacterial strains or reduced side effects. Research continues to explore these possibilities.
Spectroscopic Characterization (e.g., NMR, IR)
NMR spectroscopy provides detailed structural information. Expect to observe distinct signals for the various protons in doxycycline hyclate, reflecting their chemical environments. The presence of characteristic peaks for the dimethylamino group and the various hydroxyl and methoxy groups is particularly helpful for confirmation. Careful analysis of coupling constants will further aid in assigning specific proton resonances.
Infrared (IR) spectroscopy confirms the presence of key functional groups. Look for strong absorption bands corresponding to O-H stretches (broad peak), C=O stretches (strong, sharp peak), and C-O stretches (medium intensity). The presence of these specific peaks, along with their precise frequencies, can be compared to known spectra to validate the identity of doxycycline hyclate.
Combining NMR and IR data provides a robust approach to characterization. Discrepancies between predicted and observed spectroscopic data may indicate impurities or structural variations. Careful comparison with published spectra is always recommended for accurate identification.
For advanced analysis, techniques like mass spectrometry can be applied to determine the molecular weight, providing further supporting evidence for the presence of doxycycline hyclate.
Note: Specific chemical shifts and IR absorption frequencies will depend on the solvent and conditions used during the analysis. Refer to established literature for expected values.