Ampicillin’s activity primarily targets gram-positive bacteria, including Staphylococcus aureus (methicillin-sensitive strains), Streptococcus pneumoniae, and Enterococcus faecalis (certain strains). Its effectiveness against gram-negative bacteria is more limited, with activity against Haemophilus influenzae, Escherichia coli, and Salmonella species, but resistance is increasingly common.
Remember, Enterobacteriaceae resistance varies geographically and over time. Always consult current local antibiograms for accurate susceptibility data before prescribing. Beta-lactamase production significantly impacts ampicillin’s efficacy; consider alternative antibiotics if this is suspected. Penicillin-binding proteins (PBPs) also influence bacterial susceptibility.
For optimal therapeutic outcomes, accurate bacterial identification and susceptibility testing are paramount. Dosage adjustments are necessary based on factors such as patient age, renal function, and the severity of infection. Always adhere to established guidelines for appropriate duration of therapy. Close monitoring for adverse effects, including diarrhea and allergic reactions, is crucial.
- Ampicillin Spectrum: A Detailed Overview
- Gram-Positive Bacteria
- Gram-Negative Bacteria
- Factors Influencing Activity
- Clinical Considerations
- Mechanism of Action: How Ampicillin Works
- Targeting Peptidoglycan Synthesis
- Bacterial Resistance Mechanisms
- Gram-Positive Coverage: Effective Targets and Limitations
- Gram-Negative Coverage: Susceptible and Resistant Organisms
- Susceptible Organisms
- Resistant Organisms
- Factors Influencing Resistance
- Clinical Applications: Where Ampicillin is Most Effective
- Resistance and Alternatives: When Ampicillin Fails
Ampicillin Spectrum: A Detailed Overview
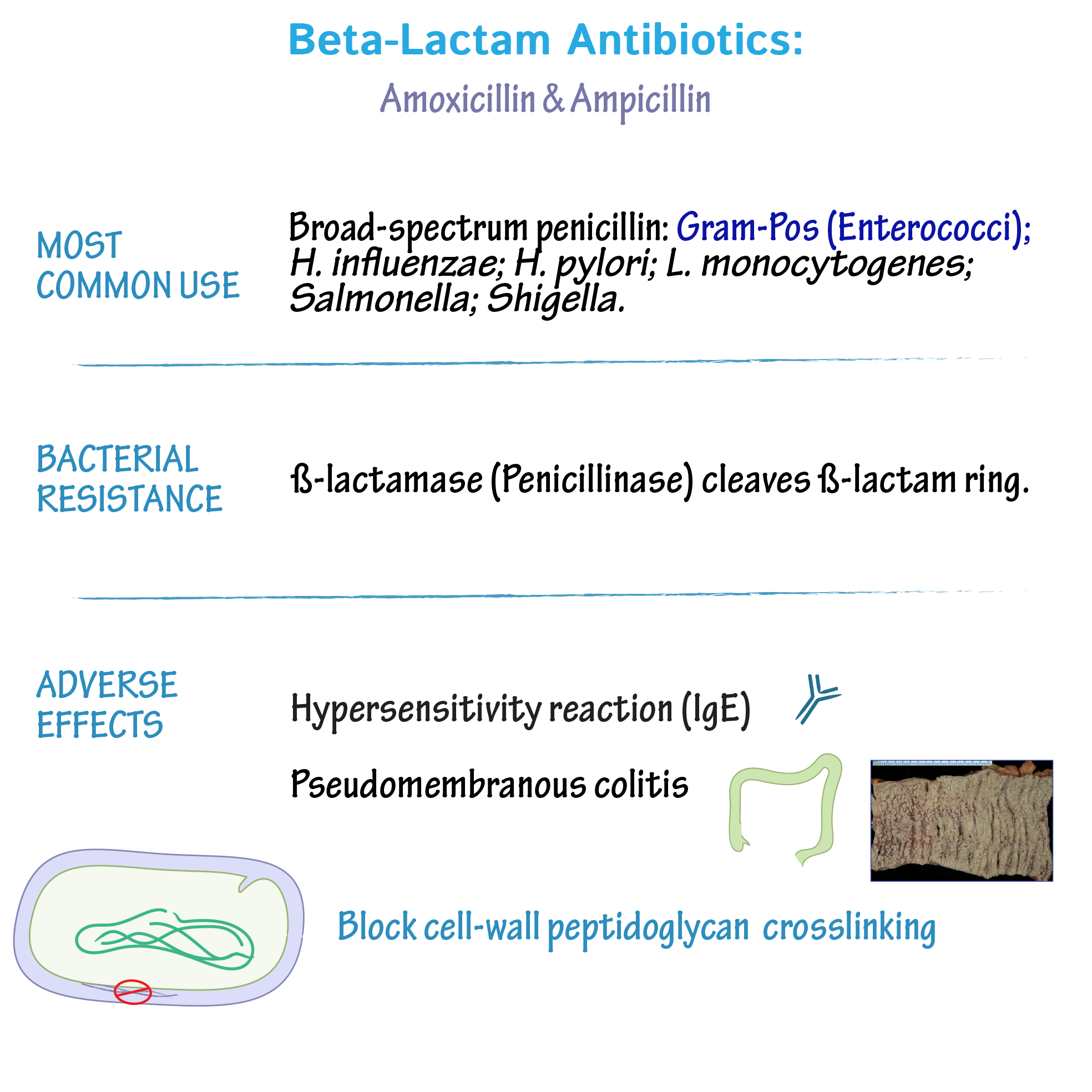
Ampicillin, a semi-synthetic penicillin, effectively targets a range of Gram-positive and some Gram-negative bacteria. Its activity stems from inhibition of bacterial cell wall synthesis.
Gram-Positive Bacteria
Ampicillin demonstrates excellent activity against many common Gram-positive pathogens. These include Staphylococcus aureus (methicillin-sensitive strains only), Streptococcus pneumoniae, Streptococcus pyogenes, and Listeria monocytogenes. However, resistance is a growing concern, especially amongst Staphylococcus species. Always check local antibiograms to guide treatment decisions.
Gram-Negative Bacteria
Ampicillin’s activity against Gram-negative bacteria is more limited compared to its effect on Gram-positive organisms. It’s generally effective against Haemophilus influenzae, Escherichia coli (certain strains), and Salmonella species. However, many strains of Pseudomonas aeruginosa, Proteus, and Klebsiella species are inherently resistant.
Factors Influencing Activity
Several factors influence ampicillin’s effectiveness. Beta-lactamases, enzymes produced by bacteria, can inactivate ampicillin, rendering it ineffective. This is a major mechanism of resistance. The concentration of ampicillin at the infection site is also crucial. Poor drug penetration into certain tissues can limit its efficacy. Finally, the bacterial strain’s inherent susceptibility plays a significant role; antibiotic sensitivity testing is always necessary.
Clinical Considerations
Ampicillin is frequently used to treat infections such as pneumonia, meningitis (caused by susceptible strains), urinary tract infections, and endocarditis (in specific cases). Before prescribing, consider the potential for allergic reactions. Monitor patients for side effects, which can include diarrhea, rash, and nausea.
Mechanism of Action: How Ampicillin Works
Ampicillin, a β-lactam antibiotic, disrupts bacterial cell wall synthesis. It achieves this by inhibiting penicillin-binding proteins (PBPs). These PBPs are enzymes vital for the cross-linking of peptidoglycans, the major components of bacterial cell walls.
Targeting Peptidoglycan Synthesis
By binding to PBPs, ampicillin prevents the formation of peptide cross-links in peptidoglycan. This weakens the cell wall, making bacteria susceptible to osmotic lysis and ultimately leading to bacterial cell death. The specific PBPs targeted vary depending on the bacterial species.
Bacterial Resistance Mechanisms
Bacterial resistance to ampicillin frequently stems from the production of β-lactamases. These enzymes hydrolyze the β-lactam ring of ampicillin, rendering it inactive. Other resistance mechanisms include alterations in PBPs, reducing ampicillin binding affinity, or changes in cell wall permeability, hindering ampicillin entry into the bacterial cell.
Gram-Positive Coverage: Effective Targets and Limitations
Ampicillin reliably targets many Gram-positive bacteria, including Streptococcus pneumoniae (most strains, though resistance is emerging), Streptococcus pyogenes, and Listeria monocytogenes. It also works well against Enterococcus faecalis, but less so against Enterococcus faecium due to widespread resistance.
However, ampicillin’s usefulness is limited by several factors. Staphylococcus aureus, a significant pathogen, often exhibits resistance. Methicillin-resistant Staphylococcus aureus (MRSA) is virtually always resistant to ampicillin. Similarly, penicillinase-producing strains of many Gram-positive bacteria will also show resistance. Beta-lactamase production hydrolyzes the beta-lactam ring, rendering ampicillin inactive.
Clinicians should consider the specific bacterial species and its potential resistance profile before prescribing ampicillin. Laboratory testing to identify the bacteria and determine its susceptibility is critical for optimal treatment. Ampicillin’s success hinges on appropriate antimicrobial stewardship practices, which minimizes resistance development.
Enterococci resistance to ampicillin is a growing concern. While some strains remain susceptible, the increasing prevalence of high-level aminoglycoside resistance in ampicillin-resistant Enterococcus necessitates careful consideration of alternative treatment options. In short, ampicillin remains a valuable tool, but its application must be guided by precise diagnosis and careful evaluation of resistance patterns.
Key Considerations: Always conduct susceptibility testing. Carefully review local resistance patterns. Consider alternatives if resistance is suspected or confirmed.
Gram-Negative Coverage: Susceptible and Resistant Organisms
Ampicillin’s gram-negative coverage is limited. Its effectiveness varies significantly depending on the specific organism and its resistance mechanisms.
Susceptible Organisms
- Haemophilus influenzae (many strains remain susceptible). Regular susceptibility testing is vital.
- Neisseria gonorrhoeae (susceptibility varies geographically; always check local antibiograms). Consider alternative antibiotics for treatment.
- Listeria monocytogenes (generally susceptible; ampicillin remains a first-line treatment option).
Note that even within susceptible species, resistance is increasingly reported. Therefore, culture and sensitivity testing is paramount before initiating ampicillin therapy.
Resistant Organisms
- Enterobacteriaceae (E. coli, Klebsiella spp., Proteus spp., etc.): High rates of resistance due to beta-lactamases are common. Ampicillin is generally ineffective.
- Pseudomonas aeruginosa: Intrinsically resistant due to low outer membrane permeability and production of beta-lactamases.
- Enterococci: Resistance is widespread due to several mechanisms, including altered penicillin-binding proteins.
When treating suspected gram-negative infections, clinicians must carefully consider local resistance patterns. Consult current antibiograms and susceptibility data to guide antibiotic selection. Alternative agents, such as extended-spectrum cephalosporins or carbapenems, might be necessary for resistant organisms.
Factors Influencing Resistance
- Beta-Lactamase Production: Many gram-negative bacteria produce enzymes that inactivate ampicillin.
- Porin Changes: Alterations in outer membrane porins reduce ampicillin’s ability to enter the bacterial cell.
- Penicillin-Binding Protein Modifications: Changes in the target site of ampicillin action prevent its binding and subsequent bacterial wall synthesis inhibition.
Clinical Applications: Where Ampicillin is Most Effective
Ampicillin shines against gram-positive bacteria like Streptococcus pneumoniae (responsible for pneumonia and meningitis), Streptococcus pyogenes (causing strep throat and scarlet fever), and Listeria monocytogenes (a foodborne pathogen). It’s a reliable choice for treating infections caused by these organisms, particularly in uncomplicated cases.
Ampicillin also demonstrates efficacy against several gram-negative bacteria, including Escherichia coli (a common cause of urinary tract infections and diarrhea), Haemophilus influenzae (a leading cause of respiratory infections, especially in children), and Salmonella species (causing typhoid fever and gastroenteritis). However, its effectiveness against gram-negative bacteria is becoming more limited due to increasing antibiotic resistance.
Specific clinical situations benefitting from ampicillin therapy include:
| Infection Type | Specific Bacteria | Considerations |
|---|---|---|
| Bacterial Meningitis | Streptococcus pneumoniae, Haemophilus influenzae, Listeria monocytogenes | Often used in combination with other antibiotics. Susceptibility testing is crucial. |
| Urinary Tract Infections (UTIs) | Escherichia coli | Efficacy depends on local resistance patterns. |
| Endocarditis (in certain cases) | Streptococcus viridans | Usually part of a combination therapy regimen. |
| Sepsis | Various susceptible bacteria | Empiric therapy pending culture results. Often used as initial treatment before results are available. |
Remember, prescribing ampicillin should always be guided by culture and susceptibility testing to confirm bacterial sensitivity and avoid the promotion of antibiotic resistance. Always consult with a medical professional for proper diagnosis and treatment.
Resistance and Alternatives: When Ampicillin Fails
Ampicillin resistance is a significant clinical challenge. β-Lactamase production is the primary mechanism. Gram-negative bacteria, especially Escherichia coli and Klebsiella pneumoniae, frequently exhibit resistance.
If ampicillin therapy proves ineffective, susceptibility testing is paramount. This guides selection of an appropriate alternative. Consider these options:
For Gram-positive infections: Cefazolin, ceftaroline, or vancomycin (depending on the organism and resistance profile) are frequently used. Linezolid or daptomycin are options for serious infections resistant to other agents.
For Gram-negative infections: Extended-spectrum cephalosporins (ceftazidime, cefepime), carbapenems (imipenem, meropenem), or fluoroquinolones (ciprofloxacin, levofloxacin) are potential alternatives. However, carbapenem resistance is a growing concern. Aminoglycosides (gentamicin, amikacin) can be combined with other antibiotics in severe infections.
Combination therapy often enhances efficacy and reduces the likelihood of resistance development. For instance, combining ampicillin with an aminoglycoside might improve outcomes in certain cases, despite ampicillin’s individual failure.
Infection site influences treatment choice. Meningitis, for example, demands antibiotics capable of crossing the blood-brain barrier effectively. Empirical treatment is sometimes necessary initially, pending culture results. Adjusting treatment based on this information is crucial.
Always follow local antimicrobial stewardship guidelines, which provide recommendations for appropriate antibiotic usage and promote responsible antibiotic use to mitigate resistance development.